Difference between revisions of "Phonegap Healthcare Adapter Bluetooth Spec"
Cwdesautels (talk | contribs) |
|||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Admon/obsolete}} | ||
| + | |||
[[Category: NexJ_Express]] | [[Category: NexJ_Express]] | ||
[[category: NexJ Express PhoneGap]] | [[category: NexJ Express PhoneGap]] | ||
| Line 56: | Line 58: | ||
==== Header Section ==== | ==== Header Section ==== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==== Data Section ==== | ==== Data Section ==== | ||
===== Blood Pressure Device Specification ===== | ===== Blood Pressure Device Specification ===== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
===== Glucose Device Specification ===== | ===== Glucose Device Specification ===== | ||
| Line 132: | Line 70: | ||
Kilogram Mode | Kilogram Mode | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 19:37, 26 January 2014
NexJ Medical Peripheral Mobile Adapter Will be designed to enable NexJ's Mobile Healthcare solutions to interact with Bluetooth peripherals.
- Main article: Mobile Medical Device Integration
Contents
Bluetooth Details
Bluetooth Profile
Serial Port Profile (SPP).
Bluetooth protocols
RFCOMM and Service Discovery protocols.
Pairing
Secure Simple Pairing.
Data transmission
Measurement date/time, measurement values, Bluetooth Id of remote unit, mode, and serial number of A&D PBT Series.
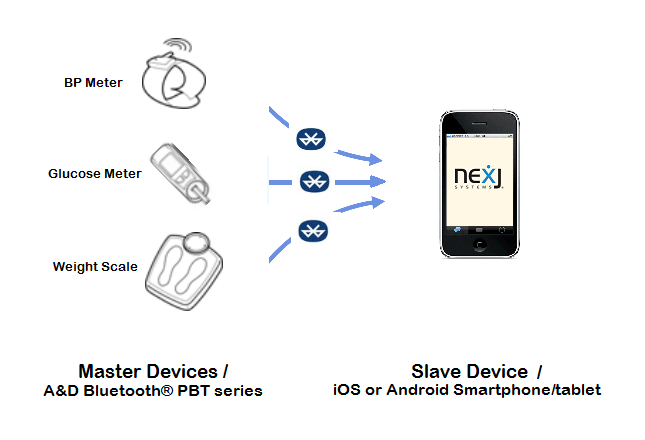
Master devices
A&D Bluetooth® devices PBT series, including blood pressure meter, blood glucose meter, and weight scale.
Slave devices/Access Points
mobile devices, including iOS and Android smartphones/tablets.
Process Description of Bluetooth Communication
Process Description for unpaired devices
- Enable Bluetooth on the mobile device and set the device to discoverable mode (as slave device).
- Make a measurement on selected A&D Bluetooth device such as weight scale. Upon the completion of measurement, the device (as master device) searches slave devices and initials Bluetooth communication.
- The mobile device receives the signal of the A&D Bluetooth device and prompts user to enter a PIN/passkey.
- Once the PIN is matched, the A&D Bluetooth device sends the measurement data to the mobile device using the specified format such as weight scale packet followed by the Confirmation Packet Response.
- Upon the success of connection, the two devices are paired. Then PIN code is no longer needed afterward.
Process Description for paired devices
- Enable Bluetooth on the mobile device and set the device to discoverable mode.
- Make a measurement on selected A&D Bluetooth device. Upon the completion of measurement, the A&D Bluetooth device checks its memory for previously paired address of mobile device and directly sends the measurement data to the mobile device using the specified format, followed by the Confirmation Packet Response.
Communication Packet
Communication packet consists of two sections: the dataheader section and the data section.
Header Section
Data Section
Blood Pressure Device Specification
Glucose Device Specification
unknown
Weight Scale Specification
Kilogram Mode